No Library Card Needed
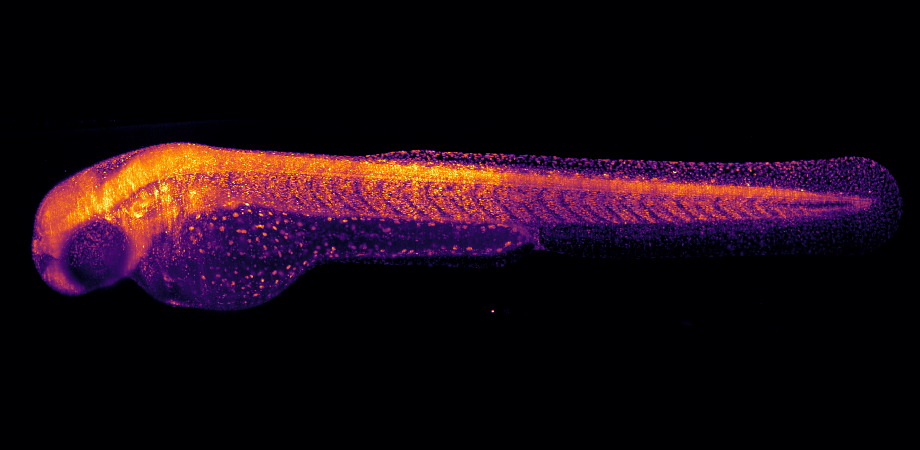
Mary Halloran's lab studies zebrafish embryos to determine how their nervous system develops, including axon and neuron branching patterns. For Halloran, a professor at the University of Wisconsin-Madison's Department of Integrative Biology, light sheet microscopy is the only tool for the job. Whereas traditional confocal imaging zooms in on just a small region of an embryo or nervous system, light sheet imaging allows them to zoom out and look at the entire embryo rapidly, with a high resolution close to the quality of a higher magnitude objective.
Unfortunately for Halloran, whose lab has just five to seven full-time people, commercial light sheet microscopes are prohibitively expensive. Fortunately for Halloran, there is now an option for labs like hers.
A new light sheet microscope, developed at the Morgridge Institute for Research, a private, nonprofit biomedical research institute at the University of Wisconsin-Madison, uses compact light engine (CLE) technology from TOPTICA Photonics, Inc. to create a system so small and portable it can easily travel to users. Its flexible modular setup and portability is the basis for a new microscope lending initiative called INVOLV3D, where collaborators can request fleets of light sheet microscopes to be sent out for research on a temporary basis.
Applications like Halloran's fragile, fluorescently labeled samples are perfect for light sheet fluorescence microscopes (LSFM). Their high speed and low photo- toxicity are used to create 2D or 3D images of living structures such as cells, tissues, or other biological organisms. LSFM shines laser light on a specimen from the side and exposes only a thin volume of a sample around the plane of interest. Fluorescence is collected with a sensitive camera to optically section a sample. Because of its low-power lasers and exposures in the millisecond range, it's a gentle high-speed technique with less toxicity, photobleaching, and damage to tissues than traditional confocal imaging methods.
Confocal microscopes, which are well-established in labs, rely on beam scanning to raster across an entire sample. This method is slow and inefficient, and over time fluorescence fades away. After hours of imaging, cells, tissues, or embryos show the effects of laser exposure. 3D imaging multiple planes is problematic as light always shines through the entire specimen perpendicular to the image plane, exposing every plane to the light.
The advantages of LSFM are quite clear. Because they cause less damage to tissues, experiments using LSFM can run for much longer. It's possible to image developmental processes in intact small animals, keeping organisms alive for many days with the right sample preparation. Wide-field objectives and the latest sCMOS or EMCCD camera technologies can be used to capture fast phenomena in the focal plane, like a beating heart or neuronal activity, or rapidly acquire an entire stack of images in a living embryo.
• • •
Typically, universities need to use advanced fluorescence microscopes only for a few weeks to gather substantial data. A handful of research groups can afford to purchase commercial light sheet microscopes, and a number can build their own. In between, there are significant numbers who don't have time, expertise, or knowledge to build one, or don't yet have funding to buy one. This new lending library initiative creates a bridge for those users.
Jan Huisken, director of medical engineering at the Morgridge Institute for Research and visiting professor in integrative biology at the University of Wisconsin-Madison, is one of the developers of light sheet microscopy technology and the father of the Flamingo Project. Researchers at Huisken's Lab use LSFM to study tissue dynamics at the cellular level of biological processes in the early development of
zebrafish and other organisms.
Huisken says the challenge for most microscopists is disseminating developed technology. After building a novel tool on an optical table in a research lab, publishing results, and getting publicity, typically no one can use the tool other than those at the lab or those willing to pack their specimens and come visit.
"Many biologists wish they had that recently published microscope in their own lab or could travel to use it. Unfortunately, we are not well equipped to host outside samples in our microscopy lab. With a modular, customizable, and portable framework we can now bring the latest technology to the biologists," he says.
Together with TOPTICA, he incorporated a CLE into a modular system developed as a traveling microscope framework and whimsically named it Flamingo, because it looks like the microscope is standing on one leg.
Light sheet microscopes allow users to build up volumetric data sets from very thin slices and create time lapse images over long periods of time, so laser robustness and stability at low power are critical. The CLE offered by TOPTICA is thermally robust and has well-known stability. It is integrated into a variety of OEM products where stability is critical, and is popular in light sheet microscopes.
Its frequency-doubled diode laser technology creates four color wavelengths in one integrated device, making a compact efficient light source that works up to 50 mW without producing a lot of waste heat or needing an external modulator.
Huisken says, "We found the CLE to be perfect for the Flamingo. It has the right power, all the laser lines needed to excite the typical fluorophores in biology, and it is robust and small enough for a compact travel microscope. TOPTICA was an obvious partner, and their service department helped integrate the components into a scope from the software side as well. They were a natural fit for us."
Whereas typical optical setups built on breadboards and optical tables aren't easily packaged or intended to move, the Flamingo prototypes are compact enough to travel to the user by car or plane, and the microscope, electronics, and minicomputer fit in two suitcases.
It's not only portable, it's also configurable. Biologists now realize the importance of looking at cells in the context of intact tissues, as opposed to extracted on a cover slip. The Flamingo system's configurability allows researchers to look deeper into intact tissues and use larger samples and a greater variety of specimens than traditional microscopes, which are limited to flat small samples.
Flamingo provides the ability to turn the lenses or the whole scope around to look at samples from the top, side, or bottom. This new flexibility for the scope and samples means biologists can get perfect images of not only fixed tissues, but things that are alive, growing, and moving around. Whole organism imaging has been a huge driver in the field.
There are other ways to customize, beginning with the lenses. There are a variety of objective lens configurations in LSFM, ranging from one to four lenses for illumination and detection. Lenses are chosen to fit desired sample sizes into the field of view with the desired magnification, numerical aperture, and working distance. Options also exist for illumination, filters, and cameras needed to match the objective lenses to have enough FOV, pixel size, sensitivity, and frame rates for various applications.

Flamingo light sheet microscope in a zebrafish room at Harvard University. Credit: Michael Weber, Jan Huisken
• • •
Huisken wants INVOLV3D, his lending library initiative of which Flamingo is the first instrument, to remain a collaborative initiative. He believes sharing benefits the community and has no plans to commercialize the Flamingo technology.
"Sharing also makes sense from a funding perspective, since tools aren't sitting in a basement unused after an experiment is completed. And from a scientific basis, getting tools into the field allows for rapid adoption of user feedback to make improvements to make everyone happy," says Huisken.
Flamingo is built with high-end lasers, stages, cameras, and opto-mechanical components, but is packaged differently from typical experimental setups. It's not spread out on an optical table but is wrapped around a central post. Several Flamingos could be built for the same cost of a single commercial system, which can cost three to eight times more, depending on the configuration. "The support of several manufacturers helped us reduce the costs," he explains.
The instrument also helps reproduce scientific results produced with other instruments. "Most journal readers don't have or can't afford to purchase expensive commercial systems. Anyone can request the same Flamingo configuration to reproduce published papers using it," Huisken says.
Creating a tool that can be passed back and forth between labs, like Flamingo, has shown there's a different way to collaborate. "Alternatively to commercializing the technology or making it open source, making multiple copies of the technology, sharing it, and allowing people to test it can be better than conventional models for the community," he says.
Thanks to close proximity to Huisken, Halloran's lab at the University of Wisconsin was one of the first to benefit from Flamingo. "We've done a lot of light sheet imaging with the Flamingo in its standard configuration holding one sample in a capillary tube, and created lots of data-rich movies with this setup. Huisken's lab helped design software for us to provide quick automatic quantitative feedback while imaging. We're still collaborating and beta testing the new setup, and we expect it will be extremely useful to look at a large set of genes rapidly and do something we couldn't otherwise do," she says.
For Duygu Özpolat, principal investigator and cell and developmental biologist at the Marine Biological Laboratory in Woods Hole, Massachusetts, the Flamingo's ability to travel and low photo toxicity solved her biggest problems. Her lab used the Flamingo for a couple weeks to image live segmented worms similar to earthworms and test different configurations.
"Normally a lab like mine couldn't purchase equipment like this since it's too expensive. We'd need to try to find someone to share and visit their tool. But that's not possible with the samples we have. It's not easy to carry animals around to places where microscopes are and keep them alive. It's nice when a high-end tool can come visit your lab. Not having to travel is a big advantage, and our most important reason for using it," she says.
Her initial impressions were that it is flexible, easy to use without being a microscopy expert, and the system's light didn't cause toxicity, alter biological processes, or damage while imaging large areas. "Light sheet scopes image samples much faster than confocal scopes. Typically it takes five minutes to go through a 60-micron-thick sample with a confocal scope. But with light sheet, you go through the same thickness in seconds. This made it possible to visualize a thicker portion of an animal without the fluorescence dimming and image for longer," says Özpolat.
As of the beginning of 2020, there are five systems in use in the field-three in Madison and two in Boston, where another team member lives. He travels with the microscope to different labs around the area to facilitate collaborations and reports back on how it works and how to improve it. While the Flamingo is the instrument they plan to replicate, the team can come up with new designs if needed.
The group will continue to use these systems for the coming years in their own lab and in many exciting collaborations. Pending funding, Huisken hopes to build many more Flamingos in 2020. He is seeking partners willing to collaborate and funding opportunities to make more systems, and already has a long waiting list of labs interested in trying the system.
In the future, Huisken envisions the microscopes will travel to remote field locations, like volcanos and oceans, without support infrastructure. He hopes to obtain grants in other countries and find partner labs to help build systems there for more widespread use around the world.
Jan Huisken would like to acknowledge support for the Flamingo Project by the Morgridge Institute for Research, the Chan Zuckerberg Initiative, Toptica, PCO, PI, and Nikon.
Debbie Sniderman is CEO of VI Ventures LLC, an engineering consulting firm.
| Enjoy this article? Get similar news in your inbox |
|



